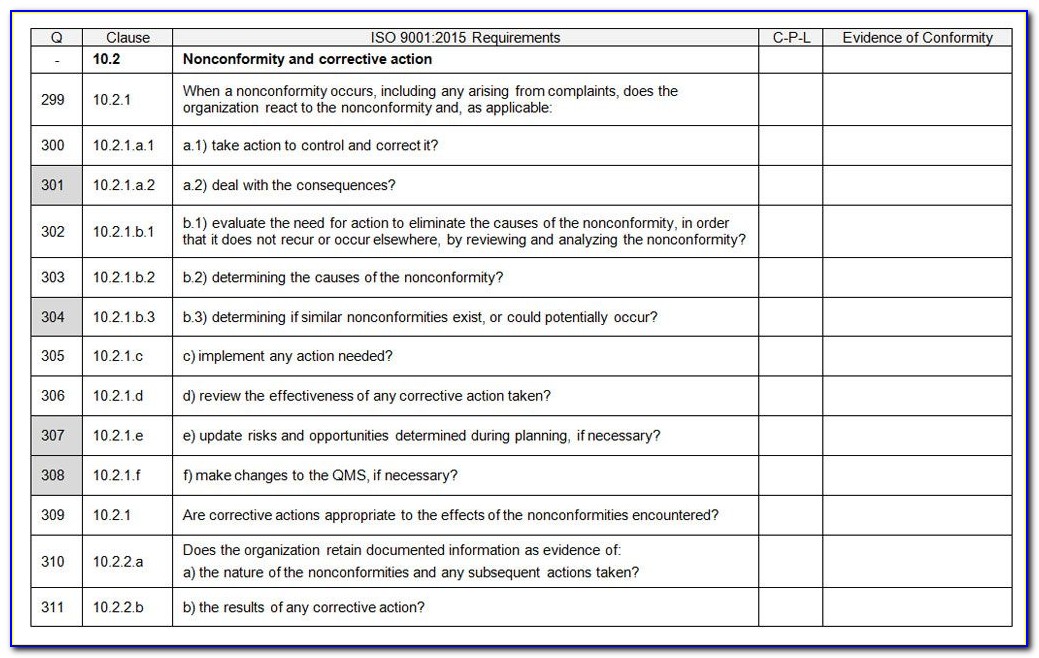
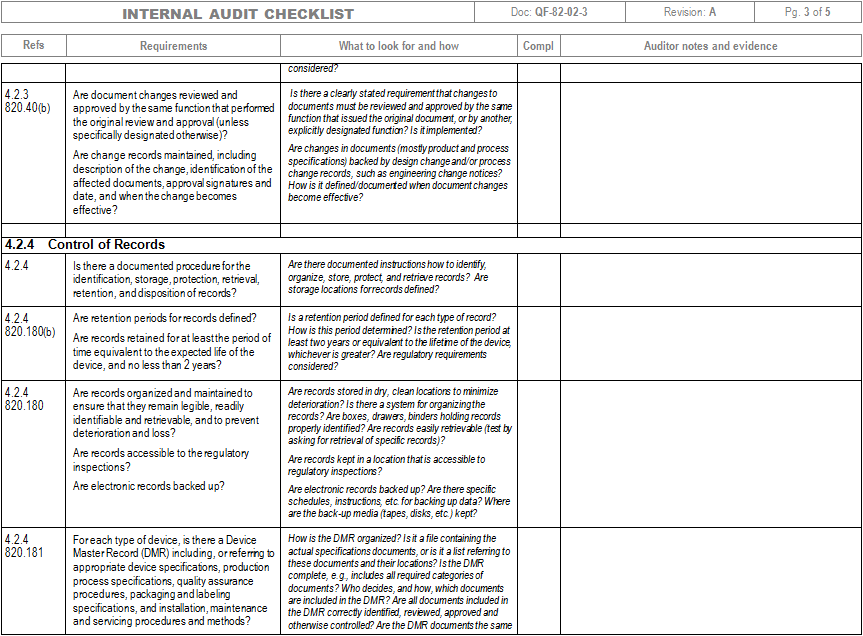
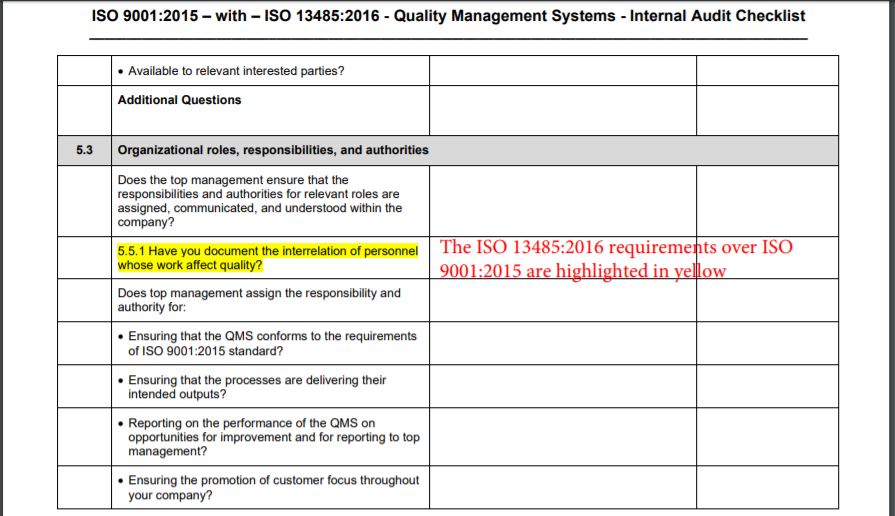
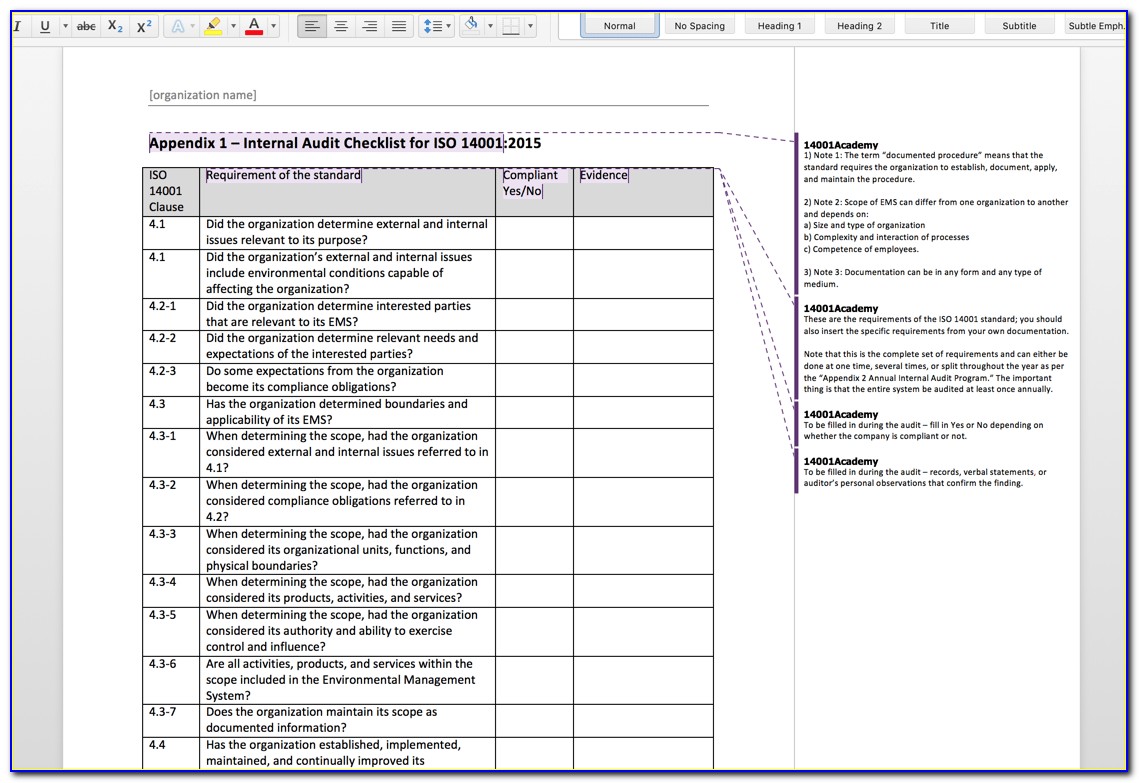
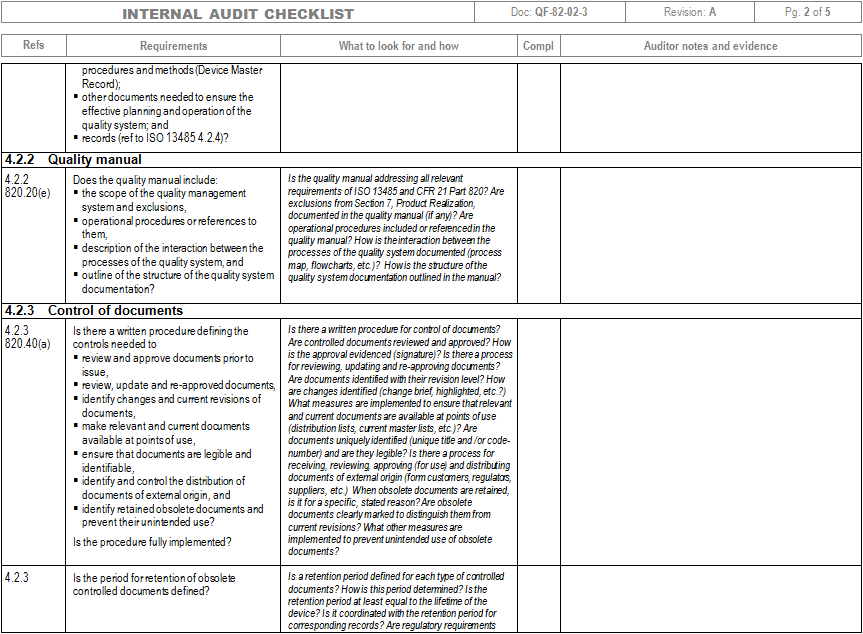
Iso 13485 Audit Checklist Template
Iso 13485 Audit Checklist Template - Here are all our posts. Document templates contain an average of twenty comments each, and offer. Conduct effective audits and inspections with the free checklist template of iso 13485 quality. Has implemented 4.2.1(a), quality 5.1(b), policy (c), 5.3, and 5.4.1) quality objectives? Ensure that you have a clear understanding of the requirements of iso 13485 to effectively use it, too. Web forms and checklists are used to record data, capture information, and facilitate compliance with iso 13485 requirements. Web looking to make your own or download iso 13485 audit checklist template to view all the tasks required and tick off the tasks when completed? Web use this iso 13485 internal audit checklist template to determine whether the company's quality management system (qms) is compliant with the iso standards. Web iso 13485:2016 standard checklist. Part 11 and validation audits.
Iso 13485 internal audit checklist advfaher
Web the documentation template may be used for iso 13485 certification audit purposes. Drug, supplements, medical device, cosmetics, 21 cfr part 11. Part 11 and validation audits. Familiarize yourself with the checklist to understand the questions or criteria it includes. The quality manual defines the scope of your qms and its procedures.
Iso 13485 Internal Audit Checklist
This iso 13485:2016 standard checklist can help quality managers find gaps in the organization’s current processes. Web iso 13485 compliance checklist pdf. Drug, supplements, medical device, cosmetics, 21 cfr part 11. Has implemented 4.2.1(a), quality 5.1(b), policy (c), 5.3, and 5.4.1) quality objectives? Web the documentation template may be used for iso 13485 certification audit purposes.
Free ISO 13485 Audit Checklists PDF SafetyCulture
The checklist is best used by trained and practicing. Has implemented 4.2.1(a), quality 5.1(b), policy (c), 5.3, and 5.4.1) quality objectives? Here are all our posts. Familiarize yourself with the checklist to understand the questions or criteria it includes. Drug, supplements, medical device, cosmetics, 21 cfr part 11.
Iso 13485 Audit Checklist 2016 doctorazgard
Web updated november 22, 2022 iso 13485 templates dr. Ensure that you have a clear understanding of the requirements of iso 13485 to effectively use it, too. Web use this iso 13485 internal audit checklist template to determine whether the company's quality management system (qms) is compliant with the iso standards. Firm has established quality audit procedures and conducts. This.
ISO 13485 Quality Management and Document Control Software Internal
These templates can include forms for. Firm has established quality audit procedures and conducts. Web iso 13485 compliance checklist pdf. Web objective parties conduct internal audits. The quality manual defines the scope of your qms and its procedures.
Iso 13485 audit checklist
This iso 13485:2016 standard checklist can help quality managers find gaps in the organization’s current processes. Web iso 13485:2016 standard checklist. Web forms and checklists are used to record data, capture information, and facilitate compliance with iso 13485 requirements. Familiarize yourself with the checklist to understand the questions or criteria it includes. The checklist is best used by trained and.
Audit Checklist For ISO 13485 Audit Business
Document templates contain an average of twenty comments each, and offer. Web updated november 22, 2022 iso 13485 templates dr. Web iso 13485 compliance checklist pdf. Web use this iso 13485 internal audit checklist template to determine whether the company's quality management system (qms) is compliant with the iso standards. Web objective parties conduct internal audits.
Iso 13485 Internal Audit Schedule Template Gambaran
These templates can include forms for. Part 11 and validation audits. The quality manual defines the scope of your qms and its procedures. Web iso 13485 compliance checklist pdf. Has implemented 4.2.1(a), quality 5.1(b), policy (c), 5.3, and 5.4.1) quality objectives?
ISO 13485 2016 Internal Auditor Checklist TQS Inc.
Web iso 13485:2016 standard checklist. Iso 13485 lays out the broad quality requirements for the modern medical device quality management system. Web iso 13485 compliance checklist pdf. Web the documentation template may be used for iso 13485 certification audit purposes. Conduct effective audits and inspections with the free checklist template of iso 13485 quality.
Iso 13485 Internal Audit Checklist
Web an mdsap audit checklist is a tool used by quality managers to determine if the manufacturer’s qms meets the requirements of iso 13485:2016 and that of. Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Web forms and checklists are used to record data, capture information, and facilitate compliance with iso 13485.
Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Web looking to make your own or download iso 13485 audit checklist template to view all the tasks required and tick off the tasks when completed? Document templates contain an average of twenty comments each, and offer. Web there are two checklist template builders available — iso 13485 audit checklist and iso 13485 standards checklist. Web an mdsap audit checklist is a tool used by quality managers to determine if the manufacturer’s qms meets the requirements of iso 13485:2016 and that of. Ensure that you have a clear understanding of the requirements of iso 13485 to effectively use it, too. Web iso 13485:2016 standard checklist. Iso 13485 lays out the broad quality requirements for the modern medical device quality management system. Has implemented 4.2.1(a), quality 5.1(b), policy (c), 5.3, and 5.4.1) quality objectives? Web use this iso 13485 internal audit checklist template to determine whether the company's quality management system (qms) is compliant with the iso standards. Conduct effective audits and inspections with the free checklist template of iso 13485 quality. The quality manual defines the scope of your qms and its procedures. Web updated november 22, 2022 iso 13485 templates dr. Web forms and checklists are used to record data, capture information, and facilitate compliance with iso 13485 requirements. Web the documentation template may be used for iso 13485 certification audit purposes. Here are all our posts. This iso 13485:2016 standard checklist can help quality managers find gaps in the organization’s current processes. Web an iso 13485 audit checklist is used by quality managers to determine whether the company's quality management system (qms) is compliant with the iso 13485:2016. Part 11 and validation audits. Firm has established quality audit procedures and conducts.